IVDR
As of May 26, 2022, the new EU In Vitro Diagnostic medical devices Regulation (IVDR) comes into effect. Whereas the IVD Directive was open to national interpretation, the IVDR is not and must be applied in its entirety across all EU countries.
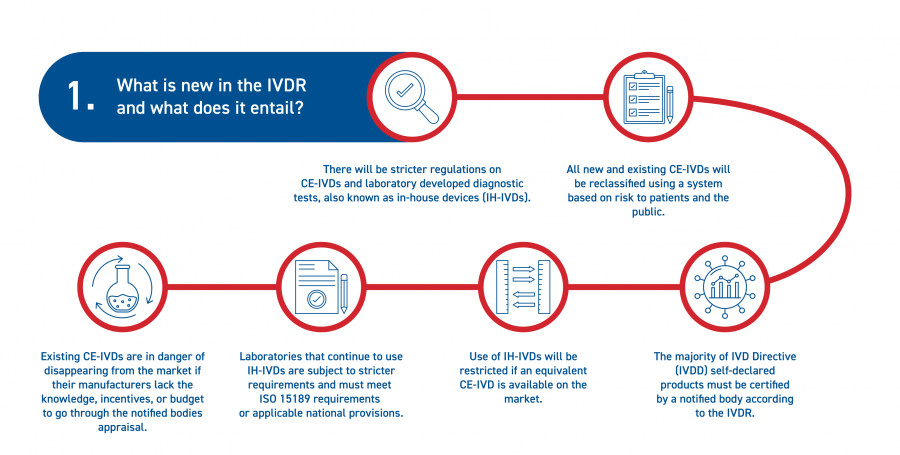
The IVDR mandates stricter and more comprehensive certification and testing protocols for in vitro diagnostic medical devices. This has a major impact on commercially available IVDs (CE-IVDs) as well as in-house devices (IH-IVDs).
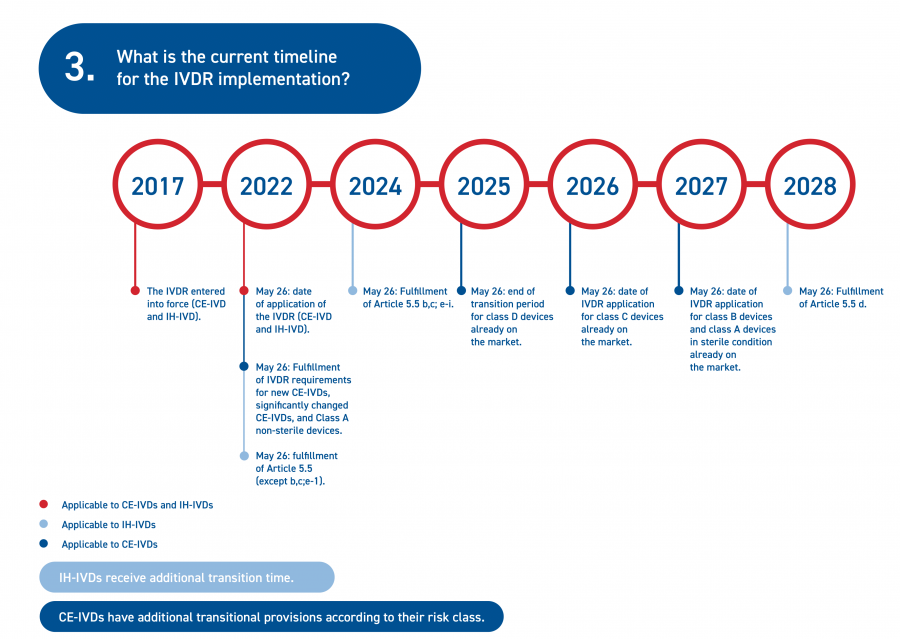
In December 2021, the Council and the European Parliament adopted a European Commission proposal amending the IVDR implementation timelines, following widespread advocacy activity. The staggered, revised implementation timelines are shown below.





 Back
Back