Regulation on Health Technology Assessment
The Regulation on Health Technology Assessment (HTAR) is legislation that was proposed by the European Commission in 2018. It was formally adopted in December 2021 and will apply from January 2025.
Scope of the HTAR
The legislation covers the clinical aspects of Health Technology Assessment (HTA). This means the relative clinical effectiveness of a new health technology, when compared with medicines or therapies already approved for the European market.
Under the HTAR, safety and effectiveness will be assessed via Joint Clinical Assessments (JCAs), conducted by the member states’ HTA bodies.
In addition, the HTAR also provides for:
- Joint Scientific Consultations, to advise technology developers on clinical study designs that generate appropriate evidence
- Horizon-scanning exercises, to identify promising health technologies at an early stage and help health systems prepare for them
Aspects of HTA not covered by the regulation
However, some aspects of HTA are outside the scope of the regulation and are reserved for individual member states. These are:
- Economic evaluation, i.e. cost-benefit assessment
- Pricing and reimbursement decisions
- Ethical and legal considerations
Foreseen benefits
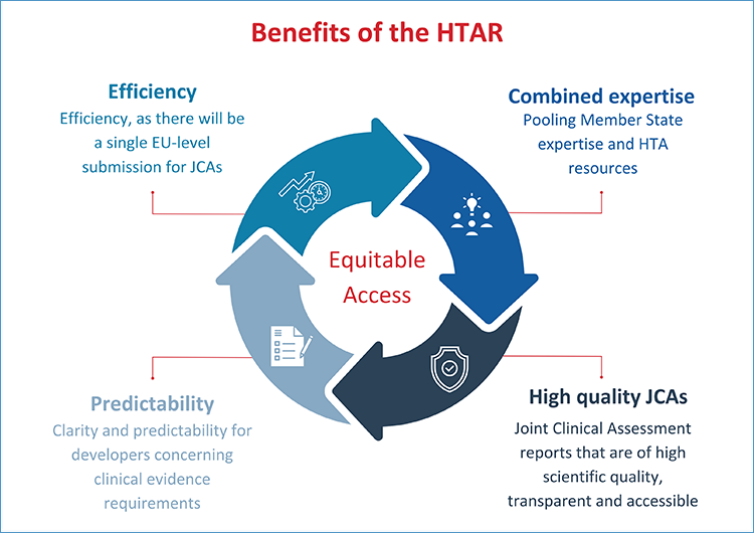
The measures in the HTAR are expected to improve:
- The pooling of member states' expertise and HTA resources
- The transparency, accessibility, and scientific quality of Joint Clinical Assessment reports
- Clarity and predictability for developers concerning clinical evidence requirements
- Efficiency, as there will be a single EU-level submission for JCAs
As a result, the HTAR will be crucial for improving timely and equitable access to innovative therapies across the EU.
Stepwise application
- January 12, 2025: new oncology medicines and advanced therapy medicinal products must comply with the HTAR.
- January 13, 2028: orphan medicinal products must comply with the HTAR.
- January 13, 2030: all new medicines will come under the scope of the regulation.
EHA involvement
EHA was heavily involved throughout the HTAR's development, and after the legislative process.
We co-led BioMed Alliance’s positioning on joint HTA, and were subsequently selected as a member of the formal HTA Stakeholder Network. This Stakeholder Network was launched in June 2023 to facilitate the involvement of stakeholders in the implementation of the regulation. It interacts directly with the European Commission and HTA bodies.
Focus of EHA's advocacy
EHA's advocacy on EU HTA [HTAR?] has focused on the following areas.
Involvement of clinical experts
In our advocacy, we have emphasized that the involvement of clinical experts should be:
- Timely
- Structural
- Meaningful
Structural and effective involvement of medical societies
We have highlighted the importance of the structural and effective involvement of medical societies, especially on the following issues:
- Expert identification and selection
- The educating and training of clinicians on HTA
- Advice on methodologies and guidance documents
- Establishing standards of care (clinical practice guidelines)
- Aligning with patient representatives and supporting their involvement
Overcoming practical challenges
As part of our advocacy work, we have identified challenges that may affect the effective involvement of clinical experts and medical societies. By extension, these issues could affect the quality and impact of EU HTA [HTAR?].
Examples of these challenges include:
- Timelines
- Conflict of Interest policies
- The facilitation and recognition of the value of clinicians' contribution to HTA by employers (such as hospitals or universities)
Harmonization
We have highlighted the need for real harmonization to make EU HTA [HTAR?] succeed, especially in:
- The formulation of PICO criteria (by national HTA bodies)
- The alignment of evidence generation (EMA-HTA-payers)
- Expert selection (not only at the national but at the European level)



 Back
Back